Last year, a project titled Nanotubular materials based on kaolin group minerals for the photodegradation of selected mycotoxins in aqueous environment received funding in the OPUS 22 competition organised by the National Science Centre. It combines issues from the crossroads of chemistry and mineralogy, which is characteristic of works in the field of applied sciences related to argillaceous minerals.
‘In the project that we’ve been working on for only six months, we want to destroy toxins using radiation and photocatalysts’, says Professor Jakub Matusik.
As the scientist claims, the most important element of photocatalysis is semiconductors, that is, reactive materials that, exposed to radiation (mostly ultraviolet), generate various radicals that can attack toxic compounds and break them up. Titanium dioxide can be a perfect example; producers add it to paints meant to remove various organic substances from the air. The semiconductors themselves, however, are not enough to combat mycotoxins; therefore, scientists want to modify natural minerals to aid the semiconductors in their struggle.
Destructive capsules
The chief mineral used in their investigations was kaolinite. It is a common argillaceous mineral, easily found in various regions, which, because of its colour and properties, is commonly used in the process of producing ceramic whiteware and paper and constitutes a filler in beauty products, giving them the desired consistency.
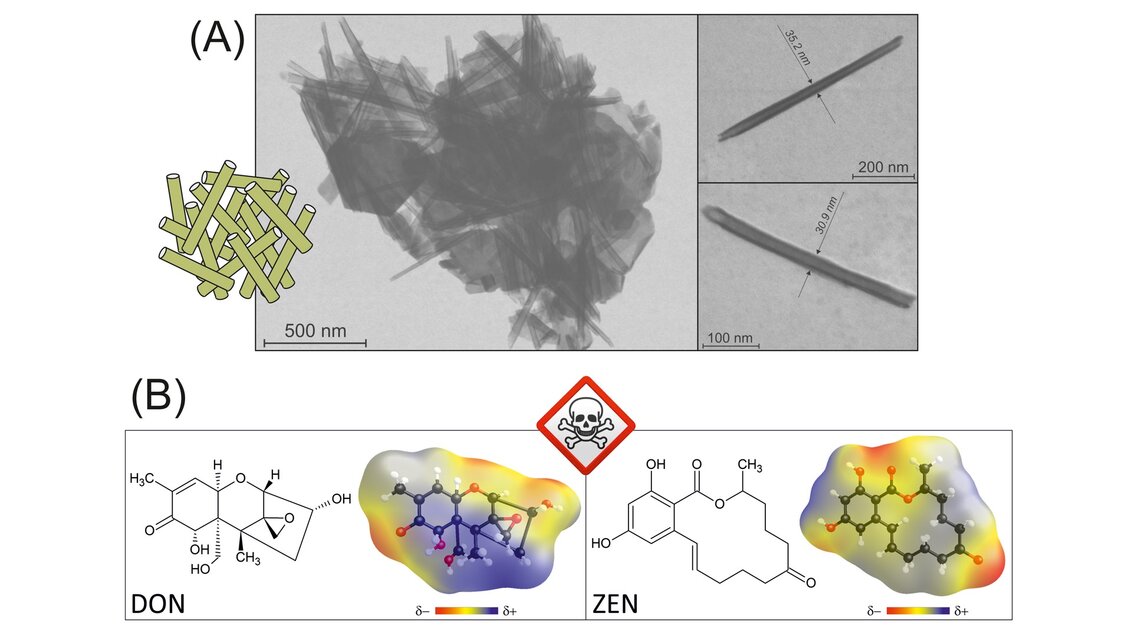
The human eye sees kaolinite in the form of a powder. Under the microscope, it naturally forms lamellar granules. While working on his doctoral dissertation, Professor Matusik managed to devise a method that allowed him to change the morphology of kaolinite plates – as a result of subsequent chemical reactions, they can roll to form tubes, the so-called ‘nanotubes’ (their length oscillates around several hundred nanometres, that is, one hundred thousandth of a centimetre). After being transformed like that, they can be used as carriers. Medicine has particularly high hopes for this solution, as nanotubes can potentially facilitate the transport of medicines and the application of substances to a specific place in the body.
‘This nanotube acts as a capsule that can transport a drug to a specific place in the organism and release it there. The release mechanisms are different and are based on, for example, the pH or the way in which a given material has been prepared, because the capsules can be sealed in different ways; various polyelectrolytes are used, for instance, that are meant to protect the medicine inside the nanotube. To the same end, we use multifarious stacked minerals: a drug is pumped between the layers that are meant to protect the particles so that it can be deployed in the right place. Especially if it is an anticancer medicine so that it doesn’t intoxicate the entire body, but acts only on the spot where it is supposed to. The nanotubular forms have the same function. There are studies related to simple particles, such as ibuprofen, but also insulin or DNA, which are also encapsulated in the structures of nanotubular or stacked minerals to transport genetic information within the organism’, says Professor Matusik about other applications for nanotubes, not directly connected to the project.
When considering this project, one significant thing is that the nanotubes can also be filled with semiconductors. Scientists expect such a mineral carrier to allow them to magnify the effect of semiconducting particles – enclosed inside a nanotubular structure, they will have a larger reaction surface with the toxins, and it can be assumed that the degradation efficiency will improve.
‘Semiconductors are placed within the tubes. Therefore, we get a limited space and when toxins penetrate the tube, they are annihilated by external radiation’, explains Professor Matusik. ‘Technically, we are testing our materials under static conditions, that is, we introduce a small weighed amount of our material in powder form into a water solution of the toxin. The reaction takes place in a sealed reactor in the presence of UV radiation. We perform the so-called "kinetic study", that is, we measure how quickly the concentration of the toxin decreases over time. In the future, we will have to determine the mechanism for the degradation of toxins’.
The process itself matters
The results obtained for the nanotubular synthetic kaolinite will be compared with the degradation efficiency of selected mycotoxins by other kaolinite materials and various semiconductors. The only constant in each combination will be the presence of ultraviolet radiation. In addition to pure plate kaolinite (used in papermaking and ceramics), the scientists will also examine halloysite, that is, a mineral from the kaolinite-serpentine group, which occurs naturally in the form of tubes. They will also use nanotubes from deposits contaminated with elements, such as iron and titanium, as well as pure variations available on the market. Professor Matusik expects that synthetic nanotubular kaolinite will yield the best results, as the process of its transformation facilitates the generation of the so-called ‘large reactive surface’ on which semiconductors react with toxins; while nanotubes from natural deposits are not always completely formed, which could limit their functionality.
What will be important during analysis is the ability to decompose mycotoxins, but not only that – the researchers have started with a detailed characterisation of materials and they use different methods to obtain as much information about them as possible: their size, structure, texture, chemical composition, and properties. They also want to check their stability, that is, determine the behaviour of materials during the degradation process; whether those materials can be reused, or whether they can be regenerated.
Sometimes, such reactions can yield unexpected results. Professor Matusik recounts an example of French scientists who research a different nanotubular mineral – imogolite:
‘They used it to decompose PAH compounds, that is, polycyclic aromatic hydrocarbons, which are also harmful compounds. The scientists observed that the degradation of these particles occurs within the tubes. These particles decompose and degrade, and simultaneously, valuable products emerge on the outer surfaces of the tubes, such as hydrogen. These observations subscribe into an interesting idea or technology known as “photoreforming”. You use waste materials and biomass or destroy harmful compounds and, at the same time, you strive to obtain valuable fuel from it – that is, simple compounds such as alcohol (e.g. methanol and ethanol) or hydrogen. This was one of my inspirations to use a material that I know so well from my doctorate, namely kaolinite, and to see how it decomposes mycotoxins, and also to use this opportunity to study various processes that are behind it. It is possible that we observe something interesting in the process, some other properties of this material, and it will lead to new ideas’.

 Pre-election meeting with a candidate for the position of rector
Pre-election meeting with a candidate for the position of rector  Agreement on cooperation with OPAL-RT
Agreement on cooperation with OPAL-RT  Krakow DIANA Accelerator consortium members with an agreement
Krakow DIANA Accelerator consortium members with an agreement  Meeting with the Consul General of Germany
Meeting with the Consul General of Germany  More Academic Sports Championships finals with medals for our students
More Academic Sports Championships finals with medals for our students  Bronze for our swimmers at Academic Championships
Bronze for our swimmers at Academic Championships  Smart mountains. AGH University scholar develops an intelligent mountain rescue aid system
Smart mountains. AGH University scholar develops an intelligent mountain rescue aid system